-
A-110
Old term and circular for Uniform Guidance Financial Status Reporting.
-
A-121
Old term for Uniform Guidance Cost Principles.
-
A-133
Old term for Uniform Guidance Single Audit, Subpart F.
-
A-21
The former Office of Management & Budget Circular A-21 regarding federal cost principles for educational institutions. This is now referred to as Uniform Guidance Monitored Costs and can be found at 2 CFR 200.
-
AAHRPP
The Association for the Accreditation of Human Research Protection Programs, Inc. (AAHRPP) is an independent, non-profit accrediting organization that uses a peer-driven evaluation process to ensure institutional HRPPs meet rigorous standards for the protections of human participants in research.
- AAHRPP Re-accreditation: All You Need to Know! Webinar slides outlining the AAHRPP accreditation process, tips on what to expect during the site visit and preparation resources. The webinar (recorded 01/26/2021) can be viewed at: https://sites.google.com/umich.edu/frankel-c3rg/past-seminar-schedule.
- AAHRPP Tip Sheets Use these brief documents from AAHRPP to review the standards and elements that form an accredited human research protection program. U-M IRBs and ORCR are encouraged to review all tip sheets.
- AAHRPP U-MIC Download the University of Michigan IRB Collaborative (U-MIC) presentation (with audio) describing the AAHRPP re-accreditation process (~ 6 minutes). Last Updated: November 2020
-
AAR
Agreement Acceptance Request
-
Accelerated Clinical Trial Agreement (ACTA)
 The University of Michigan (along with other industry partners and institutions) uses the Accelerated Clinical Trial Agreement (ACTA). For those industry partners who agree to use the agreement (and the sponsor must agree to use it), it decreases contract negotiation time. The other steps, such as budget negotiation, proposal approval form (PAF) processing, and IRB review and approval are still required.
The University of Michigan (along with other industry partners and institutions) uses the Accelerated Clinical Trial Agreement (ACTA). For those industry partners who agree to use the agreement (and the sponsor must agree to use it), it decreases contract negotiation time. The other steps, such as budget negotiation, proposal approval form (PAF) processing, and IRB review and approval are still required.HOW TO USE THIS AT U-M
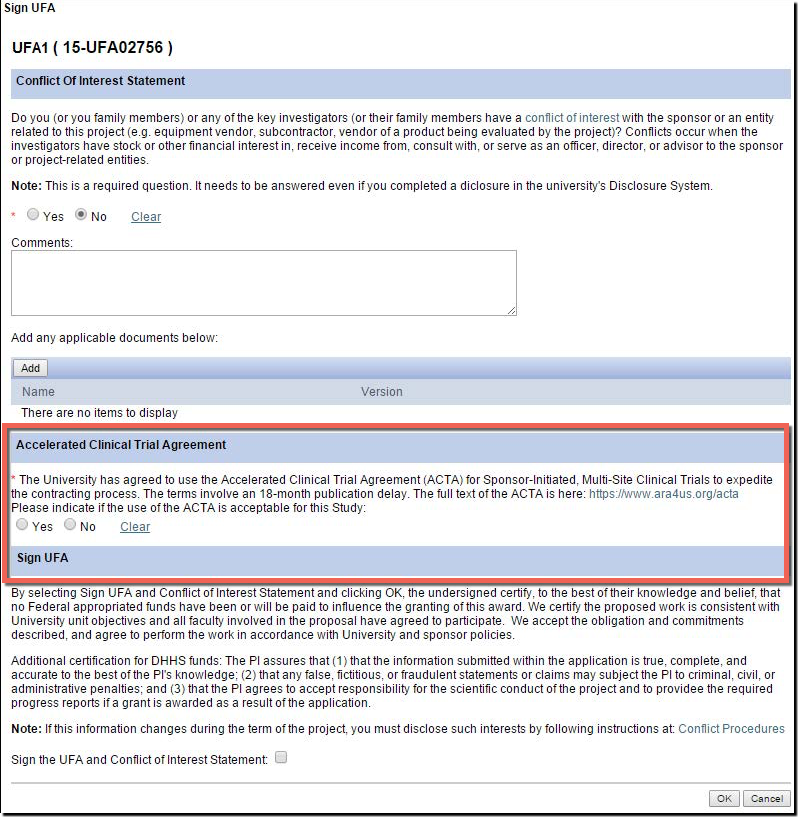
U-M supports and encourages the use of the ACTA for industry-sponsored multi-center clinical trials! Here is what you can do to take advantage:
- There is a new question in the eResearch Proposal Management System (eRPM) for the PI added to the " Sign UFA " activity where you may indicate that you would like to attempt the use of the ACTA during the UFA process.
- Answering “Yes” allows ORSP to approach the sponsor to propose the use of the ACTA.
- Additionally, on the Clinical Trials Routing Form (CTRF), the UFA answer will carry over from the NDA. If not answered, ORSP project representatives can help you later pursue the ACTA.
- Award Notices (AWDs) will also carry language to indicate if the ACTA was accepted.
- Contact one of the following ORSP PRs directly when you have questions, if a Sponsor indicates an interest in using the ACTA, or if a Sponsor would like a template agreement to review.
You can review the language in the agreement from the ACTA Website. The ACTA is not the promise of a full solution to industry-initiated clinical trial contracting, but it can help reduce one part of the process.
- There is a new question in the eResearch Proposal Management System (eRPM) for the PI added to the " Sign UFA " activity where you may indicate that you would like to attempt the use of the ACTA during the UFA process.
-
ACR - Award Change Request
Award Change Request in the eResearch Proposal Management (eRPM) system.
-
Administrative Home
The designated unit with overall responsibility for a particular proposal or project. Knowing the Administrative Home is particularly important in those cases where the Principal Investigator may have joint appointments.
-
AIHFS
American Indian Health & Family Services
-
Allowable Costs
In addition to being allowable, direct costs must follow the usual guidelines of being reasonable, allocable, applicable, and consistently treated and must comply with U-M policy.
Resources
When in doubt, please contact your Finance - Sponsored Programs Customer Service representative (see the Coordinator by Department list).
-
AOR
Authorized Organizational Representative. Often sponsors, particularly government sponsors, will ask that a task be handled or submitted by the AOR. This is the Authorized Organizational Representative and refers to an office such as the Office of Sponsored Projects (ORSP).
-
AOR
Authorized Organizational Representative. Often sponsors, particularly government sponsors, will ask that a task be handled or submitted by the AOR. This is the Authorized Organizational Representative and refers to an office such as the Office of Sponsored Projects (ORSP).
-
ARC (Advanced Research Computing)
Office of Advanced Research Computing. U-M’s ARC works to advance Cyberinfrastructure (CI) at U-M. CI refers to a platform of technological and human support for advanced, integrated computation and information resources in the service of research and learning. See http://arc.research.umich.edu/.
-
Assistance Listing Number (ALN) previously CFDA (Catalog of Federal Domestic Assistance)
The ALN was previously known as the CFDA (Catalog of Federal Domestic Assistance)
-
AST
Administrative Services Transformation (Shared Services).
-
Award
An award (or a project) results when a sponsor funds a proposal and that sponsor issues a grant, contract, or cooperative agreement to U-M. When an award is granted by a sponsor, it's time to set up the project and begin the research, managing the project through its completion or close out.
When a project is awarded, an email notification is sent to the principal investigator and the post-award administrative contact when the project/grant number is established. This serves as a notification that your project has been awarded and includes special terms and conditions associated with the award. The notification also includes information such as:
- Awarded amount
- Project/grant number and ShortCode
- Project period and budget period dates
- Funding (direct, indirect costs)
- Cost sharing, if any
- Any special terms and conditions
An award is accepted when the appropriate signatory for the university has signed the agreement and/or when you start spending on the project. You can, typically, start spending on the project when you have received the email notification of project award AND you are within the project period.
-
Award Management Functionality
A 2018 project to update the eResearch Proposal Management System (eRPM).
-
AWD
AWD refers to the Award record in the eResearch Proposal Management (eRPM) System. When an award is first activated, a system-generated email is sent to certain project team members. It provides the AWD number that helps establish the project/grant (P/G) number in the U-M accounting system for the project.
See Award Management functionality in eRPM.
Enterprise
Enterprise
As the nation’s largest public research university, the Office of the Vice President for Research (OVPR) aims to catalyze, support and safeguard U-M research and scholarship activity.
The Office of the Vice President for Research oversees a variety of interdisciplinary units that collaborate with faculty, staff, students and external partners to catalyze, support and safeguard research and scholarship activity.
ORSP manages pre-award and some post-award research activity for U-M. We review contracts for sponsored projects applying regulatory, statutory and organizational knowledge to balance the university's mission, the sponsor's objectives, and the investigator's intellectual pursuits.
Ethics and compliance in research covers a broad range of activity from general guidelines about conducting research responsibly to specific regulations governing a type of research (e.g., human subjects research, export controls, conflict of interest).
eResearch is U-M's site for electronic research administration. Access: Regulatory Management (for IRB or IBC rDNA applications); Proposal Management (eRPM) for the e-routing, approval, and submission of proposals (PAFs) and Unfunded Agreements (UFAs) to external entities); and Animal Management (for IACUC protocols and ULAM).
Sponsored Programs manages the post-award financial activities of U-M's research enterprise and other sponsored activities to ensure compliance with applicable federal, state, and local laws as well as sponsor regulations. The Office of Contract Administration (OCA) is also part of the Office of Finance - Sponsored Programs.
