You are here
- Home
- A-Z Index and Glossary
- Accelerated Clinical Trial Agreement (ACTA)
Accelerated Clinical Trial Agreement (ACTA)
-
 The University of Michigan (along with other industry partners and institutions) uses the Accelerated Clinical Trial Agreement (ACTA). For those industry partners who agree to use the agreement (and the sponsor must agree to use it), it decreases contract negotiation time. The other steps, such as budget negotiation, proposal approval form (PAF) processing, and IRB review and approval are still required.
The University of Michigan (along with other industry partners and institutions) uses the Accelerated Clinical Trial Agreement (ACTA). For those industry partners who agree to use the agreement (and the sponsor must agree to use it), it decreases contract negotiation time. The other steps, such as budget negotiation, proposal approval form (PAF) processing, and IRB review and approval are still required.HOW TO USE THIS AT U-M
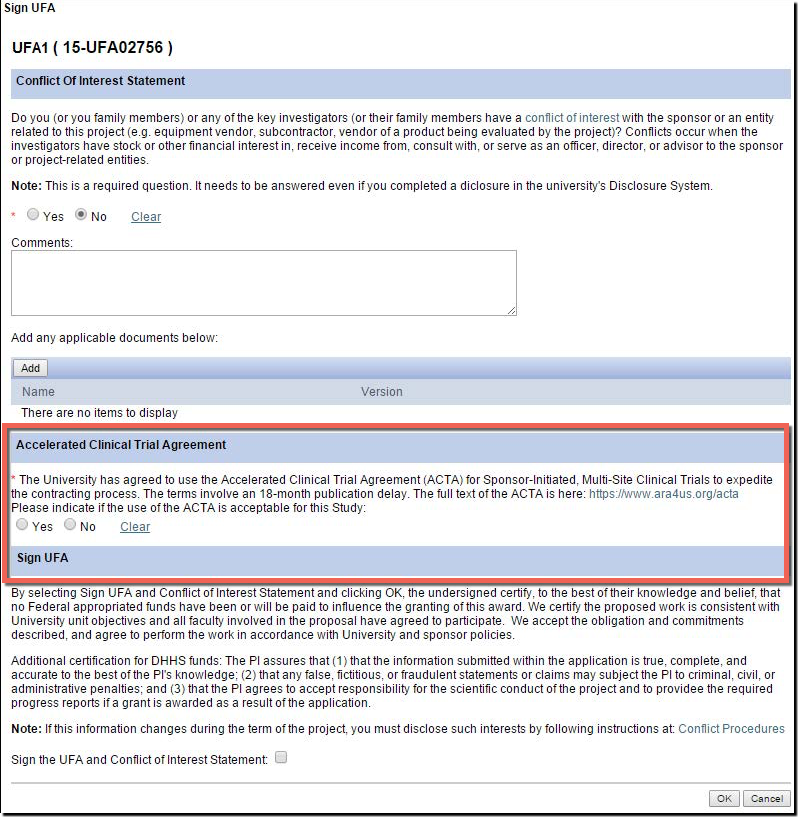
U-M supports and encourages the use of the ACTA for industry-sponsored multi-center clinical trials! Here is what you can do to take advantage:
- There is a new question in the eResearch Proposal Management System (eRPM) for the PI added to the " Sign UFA " activity where you may indicate that you would like to attempt the use of the ACTA during the UFA process.
- Answering “Yes” allows ORSP to approach the sponsor to propose the use of the ACTA.
- Additionally, on the Clinical Trials Routing Form (CTRF), the UFA answer will carry over from the NDA. If not answered, ORSP project representatives can help you later pursue the ACTA.
- Award Notices (AWDs) will also carry language to indicate if the ACTA was accepted.
- Contact one of the following ORSP PRs directly when you have questions, if a Sponsor indicates an interest in using the ACTA, or if a Sponsor would like a template agreement to review.
You can review the language in the agreement from the ACTA Website. The ACTA is not the promise of a full solution to industry-initiated clinical trial contracting, but it can help reduce one part of the process.
- There is a new question in the eResearch Proposal Management System (eRPM) for the PI added to the " Sign UFA " activity where you may indicate that you would like to attempt the use of the ACTA during the UFA process.