You are here
DURC-PEPP Policy
Policy and Guidance
U-M DURC-PEPP Implementation Workflow
Download the Implementation of the USG DURC-PEPP Policy Workflow
On May 6, 2025, a new federal policy for oversight of dual use research of concern (DURC) and certain other research with pathogens will go into effect and will apply to all federally funded research. The United States Government Policy for Dual Use Research of Concern and Pathogens with Enhanced Pandemic Potential (“USG DURC-PEPP Policy”) supersedes previous DURC policies and the 2017 Enhanced Potential Pandemic Pathogens Framework (P3CO). The USG DURC-PEPP Policy does not supersede, but complements, other existing federal regulations, including the Select Agent Regulations.
About the USG DURC-PEPP Policy
The intent of the USG DURC-PEPP Policy is to strengthen oversight of life sciences research with biological agents and toxins throughout the research lifecycle by:
- Defining an expanded scope of biological agent and toxin research subject to additional oversight by the U.S. government;
- Providing a unified framework to support the consistent identification and oversight of research proposals subject to this policy that accounts for safety, security, and ethical considerations; and
- Delineating the roles and responsibilities of principal investigators, research institutions, and federal departments and agencies that conduct, fund, or oversee research within the scope of this policy, with an emphasis on institutional oversight and management of this research.
USG DURC-PEPP Policy Statement
It is the policy of the U.S. Government that federally funded intramural or extramural research that meets the scope of Category 1 or Category 2 research within this policy is subject to federal and institutional oversight. The purpose of this oversight is to preserve the benefits of such research while minimizing the biosafety and biosecurity risks, including risks that the knowledge, information, products, or technologies generated by the research could be used in a manner that results in harm to public health and safety, agricultural crops and other plants, animals, the environment, materiel, or national security.
USG DURC-PEPP Policy Definitions
Category 1 research meets these three criteria:
- It involves one or more of the specified biological agents and toxins in the following categories. See the DURC-PEPP Category 1 List for details.
- All Federally Regulated Select Agents and Toxins including those at amounts below the Permissible Toxin Amounts.
- All Risk Group 4 pathogens listed in Appendix B of the NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines).
- A subset of Risk Group 3 pathogens listed in Appendix B of the NIH Guidelines.
- For biological agents affecting humans that have not been assigned a Risk Group in the NIH Guidelines, agents affecting humans that are recommended to be handled at BSL3 or BSL4 per the BMBL guidance are subject to the USG DURC-PEPP Policy.
- It is reasonably anticipated to result, or does result, in one of the experimental outcomes specified below:
- Increase transmissibility of a pathogen within or between host species;
- Increase the virulence (e.g. ability to cause disease) of a pathogen or convey virulence to a non-pathogen;
- Increase the toxicity of a known toxin or produce a novel toxin;
- Increase the stability of a pathogen or toxin in the environment, or increase the ability to disseminate a pathogen or toxin (e.g. environmental stability or aerosolubility);
- Alter the host range or tropism of a pathogen or toxin;
- Decrease the ability for a human or veterinary pathogen or toxin to be detected using standard diagnostic or analytical methods;
- Increase resistance of a pathogen or toxin to clinical and/or veterinary prophylactic or therapeutic interventions (e.g., antimicrobials, antivirals, antitoxins, vaccines);
- Alter a human or veterinary pathogen or toxin to disrupt the effectiveness of preexisting immunity, via immunization or natural infection, against the pathogen or toxin; or
- Enhance the susceptibility of a host population to a pathogen or toxin.
- Based on current understanding, the research can be reasonably anticipated to provide, or does provide, knowledge, information, products, or technologies that could be misapplied to do harm with no — or only minor — modification to pose a significant threat with potential consequences to public health and safety, agricultural crops and other plants, animals, the environment, materiel, or national security.
Category 2 research meets these three criteria:
- It involves, or is reasonably anticipated to result in, a pathogen with pandemic potential (PPP), or any pathogen that will be modified in such a way that is reasonably anticipated to result in a PPP.
- It is reasonably anticipated to result in, or does result in, one or more of the experimental outcomes or actions specified here:
- Enhance transmissibility of the pathogen in humans;
- Enhance the virulence of the pathogen in humans;
- Enhance the immune evasion of the pathogen in humans such as by modifying the pathogen to disrupt the effectiveness of pre-existing immunity via immunization or natural infection; or
- Generate, use, reconstitute, or transfer an eradicated or extinct PPP, or a previously identified PEPP.
- The research can be reasonably anticipated to result in the development, use, or transfer of a PEPP or an eradicated or extinct PPP that may pose a significant threat to public health, the capacity of health systems to function, or national security.
Any research that meets the definition of both Category 1 and Category 2 research is designated as Category 2 research.
Dual Use Research of Concern (DURC) is life sciences research that, based on current understanding, can be reasonably anticipated to provide knowledge, information, products, or technologies that could be misapplied to do harm with no, or only minor, modification to pose a significant threat with potential consequences to public health and safety, agricultural crops and other plants, animals, the environment, materiel, or national security.
The official designated by the research institution to serve as an internal resource for application of the USG Policy as well as the liaison (as necessary) between the institution and the relevant federal funding agency.
The entity (e.g., committee) established by the research institution to execute the institutional oversight responsibilities.
A type of pathogen with pandemic potential (PPP) resulting from experiments that enhance a pathogen’s transmissibility* or virulence, or disrupt the effectiveness of pre-existing immunity, regardless of its progenitor agent, such that it may pose a significant threat to public health, the capacity of health systems to function, or national security. Wild-type pathogens that are circulating in or have been recovered from nature are not PEPPs but may be considered PPPs because of their pandemic potential.
* "Experiments that enhance a pathogen’s transmissibility" include those that enhance environmental stability of the pathogen or toxin or change the tropism or host range of the pathogen or toxin in a way that enables an increased ability to infect and transmit between humans, among others.
A pathogen that is likely capable of wide and uncontrollable spread in a human population and would likely cause moderate to severe disease and/or mortality in humans. Pathogens with pandemic potential are often those with little to no pre-existing immunity in the human population.
“Reasonably anticipated” describes “an assessment of an outcome such that, generally, individuals with scientific expertise relevant to the research in question would expect this outcome to occur with a non-trivial likelihood. It does not require high confidence that the outcome will definitely occur and excludes experiments in which experts would anticipate the outcome to be technically possible, but highly unlikely.”
This definition captures important features of the outcome assessment that are further explained in section B3 of the DURC-PEPP Implementation Guide:
- "Relevant scientific expertise" refers to the scientific expertise required to anticipate the potential and plausible results of an experiment.
- Scientists may have differing views on possible and likely outcomes of any particular experiment, so the general assessment of multiple individuals is likely to be more robust than the views of any single individual. The PI is not required to seek assessment from a group of individuals, but rather to use the PI’s individual expertise and experience to consider the range of assessments that individuals with relevant scientific expertise would likely make.
- "Expect this outcome to occur:" While it is impossible to know for certain the result of any experiment in advance, experiments are typically conducted to test specific hypotheses. These hypotheses constitute expectations about the possible results of an experiment, and should be included in the range of results that are “reasonably anticipated” may occur. The PI may consider, if applicable, leveraging existing literature that may have analogous experimental design and/or similar pathogens or toxins to determine potential expectations.
- “Non-trivial likelihood”: A “reasonably anticipated” outcome is not necessarily the most likely outcome, nor is it necessarily an outcome with greater than 50% likelihood. Rather, it is an outcome that has a reasonable, non-negligible chance of occurring. For example, consider an experiment on pandemic influenza that experts anticipate is most likely to result in a loss of function, but that experts also believe could possibly increase transmissibility of the pathogen. An indication of generating a pandemic influenza virus with enhanced transmissibility represents a risk of high consequence to the public if that agent were to be accidentally released. Such a study should therefore undergo Category 2 oversight because, despite the fact that generating a PEPP is not the likeliest outcome, it has a non-trivial likelihood of resulting in a PEPP.
- “Excludes experiments in which an expert would anticipate the outcome to be technically possible, but highly unlikely”: For many experiments it may be possible to imagine a scenario, however unlikely, in which a genetic mutation surprisingly results in an increase in virulence or transmissibility against all reasonable expectations and prior evidence. The purpose of the Policy is to prioritize oversight for experiments that may pose the greatest risks. Technically plausible outcomes with very low likelihoods, as assessed based on pre-existing evidence, are not subject to Category 2 oversight. As per the Policy, if such a result unexpectedly arises during the conduct of research, the study should be halted, immediately be flagged for the IRE and funding entity, and be subject to Category 2 assessment and risk mitigation.
U-M DURC-PEPP Policy
The University of Michigan (U-M) adopts the USG DURC-PEPP Policy and follows the USG Implementation Guidance for identification, review, and oversight of life sciences research that is within Category 1 and Category 2 as defined by that policy.
At U-M, the USG DURC-PEPP Policy applies to all research, regardless of funding source, on the Ann Arbor, Dearborn, and Flint campuses, that meets the criteria for DURC-PEPP research. Federal sponsors may delay release of funds if a project identified as DURC-PEPP research does not fulfill all requirements for compliance with the USG DURC-PEPP Policy – this includes research that is funded or sponsored by federal grants, contracts, cooperative agreements, and other agreements.
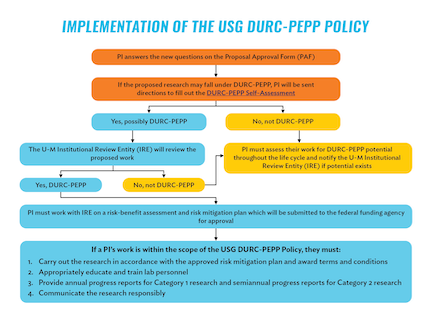
All U-M Principal Investigators (PIs) proposing or conducting research involving biological agents and toxins must assess whether or not their research potentially falls under the USG DURC-PEPP Policy. Specifically, PIs proposing to work with or generate any replication-competent infectious agent or proposing to work with a toxin of any amount from the Federal Select Agents and Toxins list must make an assessment of whether the research is reasonably anticipated to be within the scope of Category 1 or Category 2 research. If DURC-PEPP research is identified, the PI must work with the U-M Institutional Review Entity (IRE) to develop a risk-benefit assessment and risk mitigation plan that must be approved by the funding agency before the work can begin.
U-M Implementation
If a project could fall under the USG DURC-PEPP Policy, PIs will be required to complete a DURC-PEPP Self-Assessment when seeking funding for this research. The U-M Proposal Approval Form (PAF) will be updated in April 2025 with questions to ascertain whether this Self-Assessment is required, and if so, the PI and project team will be immediately notified. PIs must complete the initial DURC-PEPP Self-Assessment at the proposal stage when seeking funding.
- If identification occurs at the proposal stage, the PI must notify the federal funding agency when they submit the proposal and then contact the U-M Institutional Review Entity (IRE) to conduct the required assessments consistent with the procedures in the USG DURC-PEPP Policy for assessing Category 1 or Category 2 research.
- If identification of potential DURC-PEPP work occurs during the course of experimentation, the PI must halt further work, notify the federal funding agency, and contact the IRE to conduct the required assessments as noted above.
It is the responsibility of the IRE to then independently assesses whether the research is within scope of Category 1 or Category 2. If such work is identified, the IRE will work with the PI on a risk-benefit assessment and risk mitigation plan which will be submitted to the federal funding agency for approval. During the review process the funding agency will require the following documentation, as applicable:
- Confirmation of IRE review and category determination
- Risk-benefit assessment
- Risk mitigation plan, if research is determined to be Category 1 or Category 2
Federal funding agencies have the discretion to request additional information or to review individual research proposals or projects to determine whether they may fall within scope of Category 1 or Category 2 research.
For projects that have been identified as DURC-PEPP, reviewed by the IRE and funding agency, and received approval for the risk mitigation plan, the PI must:
- Carry out and oversee Category 1 or Category 2 research in accordance with the approved risk mitigation plan and award terms and conditions.
- Ensure that laboratory personnel conducting life sciences research within the scope of the USG DURC-PEPP Policy (i.e., those under the supervision of laboratory leadership including graduate students, postdoctoral fellows, research technicians, laboratory staff, and visiting scientists) have received and maintain education and training on all research oversight policies and processes and demonstrated competency.
- Provide annual progress reports for Category 1 research and semiannual progress reports for Category 2 research, and as requested by the federal funding agency (e.g., as part of terms and conditions of award or risk mitigation plans), for review, evaluation, assessment, and, where necessary, clarification or confirmation.
- Communicate Category 1 and Category 2 research in a responsible manner. Communication of research and research findings is an essential activity for all researchers and occurs throughout the research process, not only at the point of publication. When researchers are planning to communicate Category 1 and Category 2 research results, it is their duty to ensure that it is done in a responsible manner, and follows any measures outlined in the risk mitigation plan approved by the federal funding agency.
All PIs conducting research involving biological agents and toxins must continuously assess their work for DURC-PEPP elements throughout the research lifecycle (even if the work was not initially considered DURC-PEPP) and notify the Institutional Contact for Dual Use Research (ICDUR) if at any time before or during a project they identify potential DURC-PEPP research.
Compliance
It is the responsibility of all U-M Principal Investigators to be knowledgeable about and comply with or follow all applicable institutional and U.S. government policies, requirements, and regulations for oversight of biological agent and toxin research.
![]() For PIs and research institutions, failure to follow the USG DURC-PEPP Policy may result in suspension, limitation, or termination of federal funding and loss of future federal funding opportunities for the research proposal and for other life sciences research at the research institution, as imposed by the federal funding agency. Federal funding agencies will consider relevant statutory and regulatory authorities when considering appropriate actions.
For PIs and research institutions, failure to follow the USG DURC-PEPP Policy may result in suspension, limitation, or termination of federal funding and loss of future federal funding opportunities for the research proposal and for other life sciences research at the research institution, as imposed by the federal funding agency. Federal funding agencies will consider relevant statutory and regulatory authorities when considering appropriate actions.
The provisions of the U-M Policy on Dual Use Research of Concern and Pathogens with Enhanced Pandemic Potential are under the direction and oversight of the Assistant Vice President for Research - Research Safety Compliance (734-763-8028).
Questions?
Institutional Contact for Dual Use Research (ICDUR):
Jacqueline Shields, CPBCA
Associate Director, Research Safety Compliance
Office of the Vice President for Research
[email protected]
734-936-3934