You are here
Informed Consent Guidelines & Templates
U-M HRPP Informed Consent Information
The human subjects in your project must participate willingly, having been adequately informed about the research.
- If the human subjects are part of a vulnerable population (e.g., prisoners, cognitively impaired individuals, or children), special protections are required.
- If the human subjects are children, in most cases you must first obtain the permission of parents in addition to the consent of the children.
See the Waiver Guidelines for information about, and policies regarding, waivers for informed consent or informed consent documentation.
 IRB-HSBS has posted updated informed consent and assent templates. See the summary of changes for details.
IRB-HSBS has posted updated informed consent and assent templates. See the summary of changes for details.
See the updated Basic Informed Consent Elements document for a list of 2018 Common Rule basic and additional elements.
Informed Consent Process
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent document containing the required information (i.e., elements of informed consent) and the presentation of that information to prospective participants.
In most cases, investigators are expected to obtain a signature from the participant on a written informed consent document (i.e., to document the consent to participate) unless the IRB has waived the consent requirement or documentation (signature) requirement.
- Projects which collect biospecimens for genetic analysis must obtain documented (signed) informed consent.
- It is an ethical best practice to include an informed consent process for most exempt research. IRB-HSBS reviews, as applicable, the IRB application for exempt research, but not the informed consent document itself. A suggested consent template for exempt research can be found below under the References and Resources section. A companion protocol template for exempt research may be found in the feature box, Related Information (top right).
![]() Even in situations where the IRB may waive the documentation (signature) requirement (e.g., telephone interview, online survey), investigators are expected to present participants with the required key elements of informed consent and with a copy of the written consent document.
Even in situations where the IRB may waive the documentation (signature) requirement (e.g., telephone interview, online survey), investigators are expected to present participants with the required key elements of informed consent and with a copy of the written consent document.
Informed consent documents
An informed consent document is typically used to provide subjects with the information they need to make a decision to volunteer for a research study. Federal regulations (45 CFR 46.116) provide the framework for the type of information (i.e., the "elements") that must be included as part of the consent process. New with the revised 2018 Common Rule is the requirement that the consent document begin with a "concise and focused" presentation of key information that will help potential participants understand why they might or might not want to be a part of a research study.
Key Information Elements
The image below displays the five elements identified in the preamble to the revised Final Rule as suggested key information.
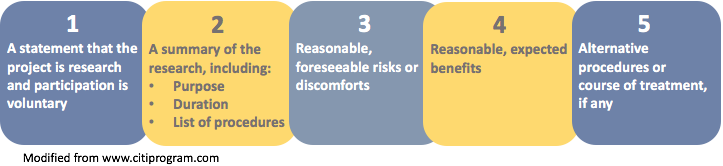
Note: Element number 5 (alternative procedures) applies primarily to clinical research.
General Information & Tips for Preparing a Consent Document
Reading level
Informed consent documents should be written in plain language at a level appropriate to the subject population, generally at an 8th grade reading level. A best practice is to have a colleague or friend read the informed consent document for comprehension before submission with the IRB application. Always:
For guidance on using plain language, examples, and more, visit: http://www.plainlanguage.gov/
- Tailor the document to the subject population.
- Avoid technical jargon or overly complex terms.
- Use straightforward language that is understandable.
Writing tips
The informed consent document should succinctly describe the research as it has been presented in the IRB application.
- Use the second (you) or third person (he/she) to present the study details. Avoid use of the first person (I).
- Include a statement of agreement at the conclusion of the informed consent document.
- The consent doucment must be consistent with what is described in the IRB application.
Document Formating for Uploading into eResearch
- Remove "track changes" or inserted comments from the consent documentation prior to uploading the document into the IRB application (Section 10-1) for review.
- Use a consistent, clearly identified file naming convention for multiple consent/assent documents.
Informed Consent Templates
IRB-HSBS strongly recommends that investigators use one of the informed consent templates developed to include the required consent elements (per 45 CFR 46.116), as well as other required regulatory and institutional language. The templates listed below include the new consent elements outlined in the 2018 Common Rule.
![]() If you choose to create an informed consent document without utilizing an IRB-HSBS template, you must ensure that all required elements are included and that the recommended language (found in the templates) is utilized appropriately.
If you choose to create an informed consent document without utilizing an IRB-HSBS template, you must ensure that all required elements are included and that the recommended language (found in the templates) is utilized appropriately.
References and Resources
Questions?
IRB-Health Sciences and Behavioral Sciences (IRB-HSBS)
Phone: (734) 936-0933
Fax: (734) 936-1852
irbhsbs@umich.edu